ABSTRACT.
It has been shown what the uniformity of the electric field, magnetic field, and gravitational field is. For the first time in the history of physics, the electric constant, magnetic constant, and gravitational constant have been compared objectively.
From the previous considerations, we know that the elementary charge carriers are an electron and positron. Therefore, the formula for electrical interaction force in the Coulomb’s law will be as follows:
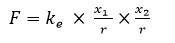
F – the attraction or repulsion force between two objects with charges,
x1, x2 – number of elementary charges in the given object,
r – distance between the objects,
ke – electric constant with a dimension [Nm2].
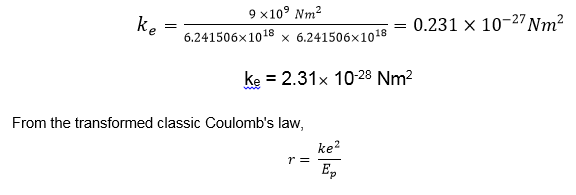
The electric constant is the force with which two elementary charges attract or repel each other from a distance of 1 m. We are able to calculate this constant. It is known that two objects with charges of 1 C attract or repel each other from a distance of 1 m with the force
9 x 109 N. It is also known that 1 C consists of 6.241506 x 1018 e (elementary charges). This means that two objects with 6.241506 x 1018 e (elementary charges) each, attract or repel each other from a distance of 1 m with the force 9 x 109 N.
Thus, the electric constant
already a long time ago we calculated the distance of an electron from proton in the hydrogen atom. It is 1.059 x 10-10 m.
Thus, now we can calculate the force with which an electron and proton attract each other in this atom
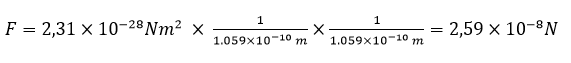
Nowadays, we are able to calculate the distance between an electron and proton and the attraction force in any electron – proton pair in any atom in the periodic table.
Here are examples.
In the periodic table, the closest to the atom nucleus is the electron neutralizing the first proton in the nucleus of uranium. Its distance from the nucleus calculated form the above classic Coulomb’s law is 1.09 x 10-14 m.
Let us now calculate the force with which the above electron and proton attract each other in this atom.
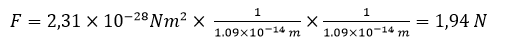
(this is an impressive force)
In the same atom, the furthest from the nucleus is the electron neutralizing the the last 92 proton in the nucleus of uranium. Its distance from the nucleus is 2.33 x 10-10 m, so the attraction force of the furthest electron is
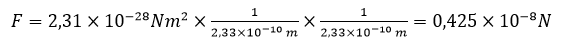
Summing up the above. In the uranium atom, the furthest from the nucleus electron is in a distance of over 21 thousand times greater that the closest one. This means that the furthest electron is attracted over 456 million times more weakly than the closest one.
From the previous considerations, we know that the elementary gravitational charge carriers are neutralized protons. The formula for the gravitational interaction in the Newton’s law will be as follows:

F – the attraction force between two objects with gravitational charges,
x1, x2 – number of elementary gravitational charges (neutralized protons) composing the given object,
r – distance between the objects,
G – gravitational constant with a dimension [Nm2].
The gravitational constant is the force with which two elementary gravitational charges attract each other from a distance of 1 m. We are able to calculate this constant. It is known that two objects of masses of 1 kg attract each other from a distance of 1 m with the force of 6.67 x 10-11 N. It is also known that one kilogram of mass consists of 0.6 x 1027 of elementary gravitational charges.
This means that two objects with 0.6 x 1027 of elementary gravitational charges (neutralized protons) each, attract each other from a distance of 1 m with the force of
6.67 x 10-11 N.
Thus, the gravitational constant

The above pair should be joined by the magnetic constant km of which we know that it should have the dimension just like the other constants, but we do not know its numerical value.
It turns out that we are able to calculate this value.
Nowadays, the following values are applied in physics:
– electric constant [9 x 10-9 Nm2/C2],
– gravitational constant [6.67 x 10-11 Nm2/kg2],
– magnetic constant [12.56 x 107 Nm2/(Am)2].
These constants are incomparable to each other because they have different titers.
A m (ampere meter) present in the dimension of the magnetic constant is the unit of “the amount of magnetism” contained in an object. A m is an equivalent to C (Coulomb) in the formula for the electric constant and an equivalent to kg (kilogram) in the formula for the gravitational constant. This unit of “the amount of magnetism” can be described in words, based on the definition of ampere.
[Am] is a number of elementary magnetic dipoles (electrons) in each meter, placed in the vacuum of two parallel, endless and infinitely thin conductors with current, in a distance of 1 m from each other, creating between them the attraction or repulsion magnetic force of 2 x 10-7 N for every meter of the length of the conductor.
If we knew the numerical value [Am], we would be able to objectively determine the magnetic constant km, just like we determined the constants ke and G. It is clearly shown from Model 31 that these elementary magnetic dipoles are electrons which apart from elementary charges are also carriers of elementary magnetic charges. It is known the magnetic moment of an electron, called the Bohr magneton, whose value is 9.27 x 10-24 Am2. This results with the fact that the amount of magnetism in each pole of the magnetic dipole of an electron is
9.27 x 10-24 Am.
Thus, we have a determined numerical value of an elementary magnetic dipole. This means that an Am unit consists of
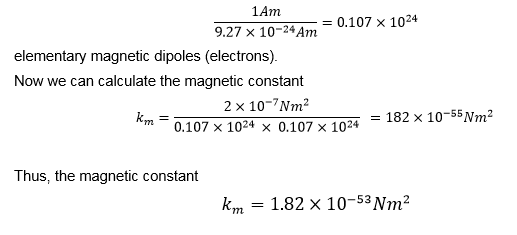
Now it is clear why we have made so much effort with the above calculations. For the first time in the history of physics, the electric constant ke, magnetic constant km, and gravitational constant G can be compared objectively.
ke = 2.31 x 10-28 Nm2
km = 1.82 x 10-53 Nm2
G = 1.85 x 10-64 Nm2
Very important conclusions derive from the above considerations.
With regard to the magnetic constant.
One ampere is not a flow of charge of 1 C in a time of 1 s.
One ampere is of elementary magnetic dipoles (electrons) in each meter, placed in the vacuum of two parallel, endless and infinitely thin conductors with current, in a distance of 1 m from each other, creating between them the attraction or repulsion magnetic force of 2 x 10-7 N for every meter of the length of the conductor.
An ampere is a unit of the same kind as a coulomb and kilogram.
1 A consists of of elementary magnetic dipoles (electrons).
1 C consists of 6.241506 x 1018 of elementary charges (electrons).
1 kg consists of 0.6 x 1027 of elementary gravitational charges (neutralized protons).
With regard to the gravitational constant.
In connection with the fact that the gravitational interaction begins to work on the level of neutralized protons, it is not justified to consider the gravitational interaction between a proton and electron. The interaction between a proton and electron can be only considered as an electric interaction between an elementary charge of an electron and an elementary charge of a positron, which is a component of the proton. Only after the occurrence of such an interaction, there is formed a neutralized proton which appears to be a carrier of an elementary gravitational charge. A neutralized proton does not interact gravitationally with an electron or positron. Neutralized protons interact gravitationally only with other neutralized protons.

